Programs

Epimutilins, A Novel Class of Oral And IV Antibacterials Overcoming Antimicrobial Resistance
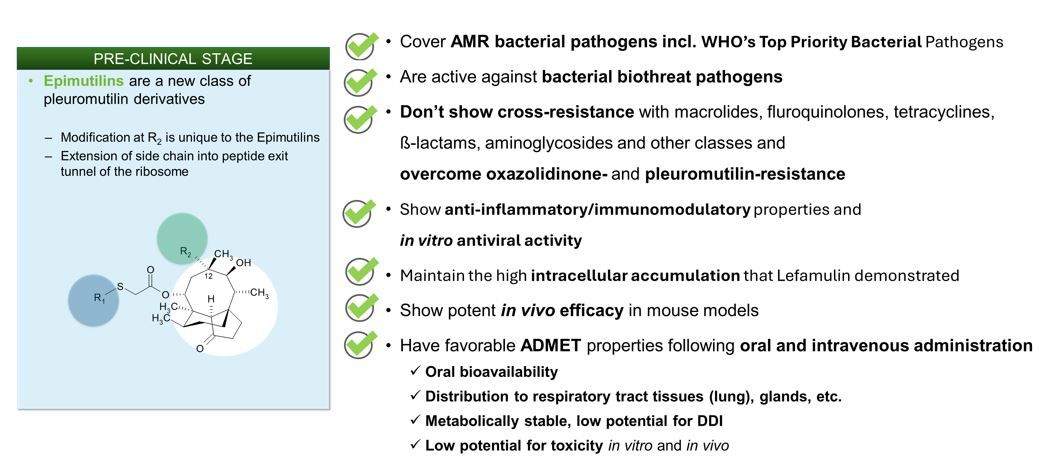
Epimutilins are outstanding novel compounds that are unaffected by antimicrobial resistance to other antibiotic classes and that show potent antibacterial activity against the most prevalent priority pathogens and global health threats as defined by the WHO and CDC.
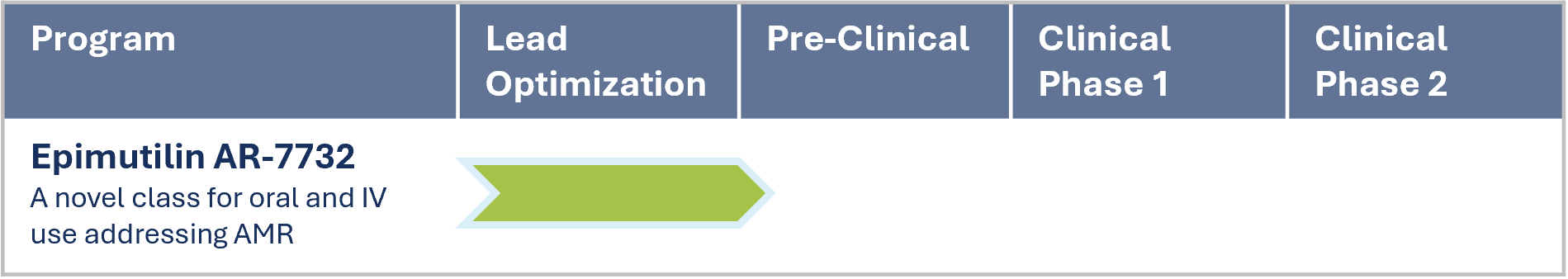
AR-7732 was selected as the lead compound among a variety of hits within the lead optimization stage. ARIVA Med is about to start the pre-clinical IND enabling studies and to profile this potent compound further.
AR-2126, A Novel Siderophore Cephalosporin
Targeting Acinetobacter baumannii, Pseudomonas aeruginosa, Burkholderia complex
Inhalation is an ideal delivery route for the treatment of lower respiratory tract infections
- AR-2126 is a novel siderophore-cephalosporin taking advantage of the iron-uptake system of Gram-negative bacteria
- AR-2126 shows potent antibacterial activity against Acinetobacter baumannii, Pseudomonas aeruginosa and Burkholderia species
- IV administration for hospitalized HABP/VABP patients
- Local delivery of AR-2126 by inhalation results in high therapeutic concentrations at the site of infection, the lung, maximizing efficacy and minimizing systemic exposure
Various lead-optimization and profiling studies including in vivo proof-of-efficacy and PK studies confirmed AR-2126 as the lead compound. Currently, ARIVA Med is about to start the pre-clinical IND-enabling program.

AR-7013, A Non-Systemic Pleuromutilin in Clinical Stage

Enormously high resistance rates among bacteria causing skin and eye infections drive the high medical need for topical agents that are unaffected by resistance
- AR-7013 is a potent antibacterial pleuromutilin
- AR-7013 is highly active against drug-resistant Gram-positive pathogens
- No cross-resistance to other antibiotic classes
- The AR-7013 pharmacology is optimal for topical treatment
- The pre-clinical program has been completed
- AR-7013 was well tolerated in phase 1 clinical trials after nasal administration

AR-7013 has demonstrated potent efficacy in vitro and in vivo, minimal systemic exposure, high local tolerance after topical administration to the skin, the eye and the GI tract in animal studies and has shown very good tolerability in human healthy volunteers in a phase 1 clinical trial.
ARIVA Med is actively looking for partners to continue the clinical development of this potent compound that will provide convenient and effective treatment options for patients with skin and eye infections caused by drug-resistant bacteria.